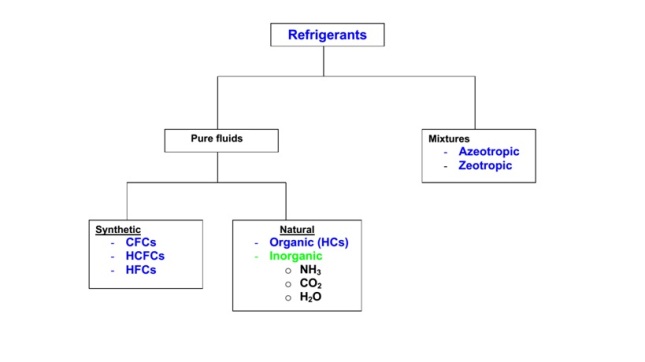
Designation of refrigerants
Since a large number of refrigerants have been developed over the years for a wide variety of applications, a numbering system has been adopted to designate various refrigerants. From the number one can get some useful information about the type of refrigerant, its chemical composition, molecular weight etc. All the refrigerants are designated by R followed by a unique number.
i) Fully saturated, halogenated compounds: These refrigerants are derivatives of alkanes (CnH2n+2) such as methane (CH4), ethane (C2H6).
These refrigerants are designated by R XYZ, where
- X+1 indicates the number of Carbon (C) atoms
- Y-1 indicates number of Hydrogen (H) atoms, and
- Z indicates number of Fluorine (F) atoms
The balance indicates the number of Chlorine atoms. Only 2 digits indicates that the value of X is zero.
Ex: R 22
- X = 0 ⇒ No. of Carbon atoms = 0+1 = 1 ⇒ derivative of methane (CH4)
- Y = 2 ⇒ No. of Hydrogen atoms = 2-1 = 1
- Z = 2 ⇒ No. of Fluorine atoms = 2
The balance = 4 – no. of (H+F) atoms = 4-1-2 = 1 ⇒ No. of Chlorine atoms = 1
∴The chemical formula of R 22 = CHClF2
Similarly it can be shown that the chemical formula of:
- R12 = CCl2F2
- R134a = C2H2F4 (derivative of ethane)
(letter a stands for isomer, e.g. molecules having same chemical composition but different atomic arrangement, e.g. R134 and R134a)
ii) Inorganic refrigerants: These are designated by number 7 followed by the molecular weight of the refrigerant (rounded-off).
Ex.:
Ammonia: Molecular weight is 17, ∴ the designation is R 717
Carbon dioxide: Molecular weight is 44, ∴ the designation is R 744
Water: Molecular weight is 18, ∴ the designation is R 718
iii) Mixtures: Azeotropic mixtures are designated by 500 series, where as zeotropic refrigerants (e.g. non-azeotropic mixtures) are designated by 400 series.
Azeotropic mixtures:
- R 500: Mixture of R 12 (73.8 %) and R 152a (26.2%)
- R 502: Mixture of R 22 (48.8 %) and R 115 (51.2%)
- R503: Mixture of R 23 (40.1 %) and R 13 (59.9%)
- R507A: Mixture of R 125 (50%) and R 143a (50%)
Zeotropic mixtures:
- R404A : Mixture of R 125 (44%), R 143a (52%) and R 134a (4%)
- R407A : Mixture of R 32 (20%), R 125 (40%) and R 134a (40%)
- R407B : Mixture of R 32 (10%), R 125 (70%) and R 134a (20%)
- R410A : Mixture of R 32 (50%) and R 125 (50%)
iv) Hydrocarbons:
- Propane (C3H8) : R 290
- n-butane (C4H10) : R 600
- iso-butane (C4H10) : R 600a
Unsaturated Hydrocarbons:
- R1150 (C2H4)
- R1270 (C3H6)